Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals
Devin Whipple, with adviser P. J. A. Kenis, Department of Chemical & Biomolecular Engineering, UIUC
The electrochemical reduction of CO2 into useful products addresses many intertwined problems in energy and climate change. This approach is more attractive for reducing CO2 emissions than capture and sequester, as no sequestration site is required and a marketable product is provided. In addition, using renewable energy such as wind and solar to reduce CO2 into products including formic acid, methanol and syngas could provide a source of renewable, net-zero-carbon-emission fuels and feedstocks for chemical synthesis. Also, coupling an electrochemical reactor for reducing CO2 with a fuel cell would allow electricity to be stored in chemical form (such as formic acid), and later reconverted to electricity. This would provide a means of energy storage necessary for intermittent renewable sources to become a major electricity source.
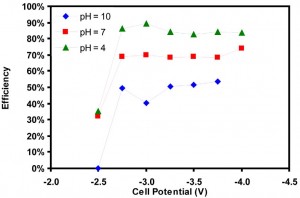
Figure 34: Reactor efficiency as a function of cell potential and electrolyte pH using 0.5M KCl, Sn Cathode and Pt Anode.
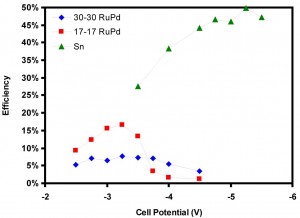
This project has focused on the development of a microfluidic reactor for the electrochemical reduction of CO2. The design uses a flowing liquid electrolyte stream, which offers a number of benefits: (i) media is flexible in both composition and pH, (ii) electrolytes supply reactant (H2O) to the anode and eliminate water management issues, and (iii) reference electrodes in the exit steam allow analysis of individual electrodes. The flexibility of the design makes the reactor an excellent tool for studying different catalysts and reactor conditions for their efficiency in CO2 reduction. Initially, this reactor was used to study several catalysts (Figure 33) and the effects of pH (Figure 34) on the conversion of CO2 to formic acid. Further study will include a more thorough investigation of reactor conditions, including electrolyte composition, pH, and temperature, as well as the production of other products such as syngas (CO and H2).
This research is supported by the Grainger Center for Electric Machinery and Electromechanics.