Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals
Ph.D. student Devin Whipple, with adviser P. J. A. Kenis, Department of Chemical & Biomolecular Engineering, University of Illinois
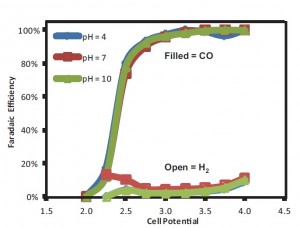
Figure30: Reactor Faradaic efficiency for CO and H2 as a function of cell potential and pH using silver nanoparticle catalyst and 1M KCl electrolyte.
The electrochemical reduction of CO2 into useful products has a number of benefits that help address many intertwined problems in energy and climate change. These benefits include: (i) a means for recycling CO2 in order to reduce its accumulation in the atmosphere, (ii) a method for producing hydrocarbon fuels from renewable electricity for use as transportation fuels, (iii) a means for storing electrical energy in chemical form for leveling electrical output from intermittent energy sources such as wind and solar.
This project has focused on using a microfluidic reactor as a platform to study CO2 electrochemical reduction. The design uses a flowing liquid electrolyte stream, which offers a number of benefits: (i) media is flexible in both composition and pH, (ii) electrolyte supplies reactant (H2O) to the anode and minimizes water management issues, and (iii) reference electrode in the exit steam allows individual electrode analysis. The design flexibility makes the reactor an excellent tool to examine different catalysts and reactor conditions for their efficiency in CO2 reduction. Previously, we have applied this reactor to compare several catalysts and have shown that decreasing pH provides significant improvements in converting CO2 to formic acid. Our current work is focused on studying how parameters such as electrolyte pH affect the production of syngas (CO and H2)—attractive because it is versatile as a feedstock for generating a wide variety of products via Fischer-Tropsch synthesis. In contrast to formic acid, syngas production efficiency is unaffected by the electrolyte pH (Figure 30). These observations are consistent with the different reaction pathways for each product. We are also investigating novel electrolytes such as ionic liquids, which show promise for decreasing the overpotential required for CO2 reduction, and carbon nanotubes used as a catalyst support and binder to increase the stability and performance of the catalyst layer.
This work is supported by the Grainger Center for Electric Machinery and Electromechanics.