Simulation of Multiple Battery Electrochemistries in a Dynamic Electric Vehicle Simulator for Energy Efficiency Comparisons
Ryan Kroeze with adviser P. T. Krein
A battery model capable of reproducing lithium-ion (Li-ion), nickel-metal hydride (NiMH), and lead-acid I-V characteristics (with minimal model alterations) has been designed and tested. The battery model was programmed into a Matlab/Simulink environment and used as a power source for plug-in hybrid electric vehicle and electric vehicle simulations. Results from simulations of Li-ion battery packs show that the proposed battery model behaves well with the other subcomponents of the vehicle simulator; accuracy of the model and prediction of battery internal losses depend on the extent of tests performed on the battery used for the simulation. Nickel-metal hydride and lead-acid battery packs have also been tested but require slight alterations of the battery model and testing methods in order to produce the same level of terminal-voltage accuracy as the Li-ion battery model.
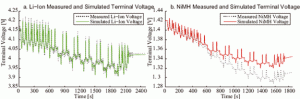
The measured and simulated terminal-voltage response to a city/highway driving schedule is shown for Li-ion batteries in Figure 8(a). Variance in the two terminal-voltage plots is minimal; greater terminal-voltage inaccuracy can be seen in Figure 1(b), the measured and simulated terminal-voltage responses for NiMH batteries. This larger error between the measured and simulated voltage occurs because measurements for the NiMH open-circuit voltage using the C/25 constant-current discharge and charge rates must be averaged; just the C/25 discharge rate was used for the Li-ion open-circuit voltage. The averaging method takes in account only an average internal resistance throughout the state of charge (SOC) range account, not resistive drops caused by larger time constants within the battery model. Open-circuit voltage accuracy can be increased for both NiMH and lead-acid batteries by averaging the C/50 constant-current discharge and charge curves. An averaging method which includes hour time-constant resistive drops will also greatly increase its accuracy.
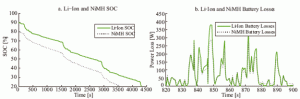
With all three battery electrochemistries validated, energy efficiency comparisons can be simulated using various driving schedules and battery pack masses and sizes. Figure 9(a) shows the SOC of 150 kg Li-ion and NiMH battery packs when simulated through the Federal Urban Driving Schedule (FUDS) velocity profile. The Li-ion battery pack was simulated through multiple FUDS, starting at 90% SOC and ending at 20%; the NiMH pack started at 80% and ended at 20%. Both battery pack power losses are shown in Figure 9(b); the Li-ion showed slightly higher losses due to higher internal resistance. Throughout the entire driving schedule, the Li-ion battery pack overall discharging energy efficiency was 93%, slightly less than the 93.8% found for the NiMH pack. The slightly higher energy efficiency seen by the NiMH battery pack is not conclusive, because the charging efficiency of both battery packs is not included in the simulation.
This work is supported by the Bitrode Corporation through the Power Affiliates Program.