Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals
M.S. student Devin Whipple with adviser P. Kenis, Department of Chemical & Biomolecular Engineering, UIUC
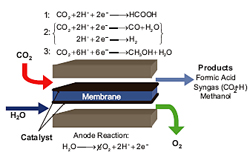
This project reduces CO2 emission by forming a useful product from it and storing electrical energy in a chemical form. The latter is important if fuel cells using formic acid and methanol are to become a backup for solar and wind power, intermittent energy sources. In the first of the two project tasks—development of an effective catalyst for CO2 reduction—we have worked on a bi-metallic ruthenium palladium catalyst to produce formic acid. Two methods for synthesizing the catalyst produce carbon-supported catalysts. They have been analyzed via cyclic voltammetry, transmission electron microscopy, and inductively coupled plasma mass spectroscopy and compared. The next step is to test the catalyst in our microfluidic cell. By changing the catalyst used we can produce products from CO2 other than formic acid, including methanol and syngas (CO and H2).
The second task is to design a rector or system capable of CO2 reduction at realistic quantities and concentrations. Work has focused on developing a microfluidic reactor that utilizes transport phenomena on the microscale to increase the reactor efficiency. The microscale allows precise control over convection and diffusion, which our design exploits to make a simpler and more efficient reactor. This reactor design also allows for media flexibility, enabling the reactor to run at either alkaline or acidic conditions and creating more opportunities for using a variety of catalysts. Much of the work has been on the critical balance between gas and liquid pressures that are necessary for optimal catalyst wetting in the reactor. The goal is to develop a reactor design capable of CO2 reduction in an efficient, cost-effective way.
This work is supported by the Grainger Center for Electric Machinery and Electromechanics.