Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals
PhD student Devin Whipple with advisor P.J.A. Kenis, Department of Chemical & Biomolecular Engineering, University of Illinois at Urbana-Champaign
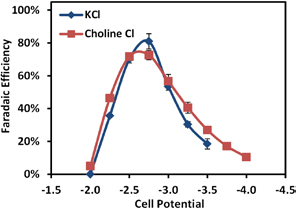
Figure 48: Reactor Faradaic efficiency (selectivity) for CO2 reduction to CO using an Au catalyst for two electrolytes: potassium chloride and choline chloride.
The electrochemical reduction of CO2 using renewable electricity has several key benefits that can help address many of the intertwined problems in energy and climate change. In particular, it recycles CO2 as an energy carrier to produce carbon-neutral fuels and provides a convenient means of storing electrical energy in chemical form. These
advantages could enable the production of alternative fuels for the transportation sector and provide a convenient method to level the electrical output from intermittent energy sources, such as wind and solar, which would
help decrease dependence on foreign oil and curb CO2 emissions.
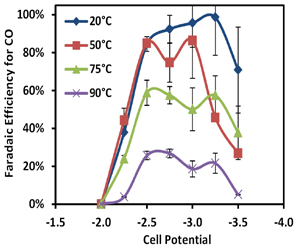
Figure 49: Reactor Faradaic efficiency (selectivity) for CO2 reduction to CO using an Ag catalyst for temperatures ranging from 20°C to 90°C.
This project has employed a microfluidic reactor as a platform for studying the electrochemical reduction of CO2. We have conducted several studies including comparing various catalysts and investigating the effects
of electrolyte pH on the conversion of CO2 to formic acid and syngas (CO and H2). Most recently, we investigated novel electrolytes for suppressing hydrogen evolution and studied the effects of temperature on CO2 reduction. We showed that a choline chloride electrolyte can suppress hydrogen evolution, which is the main competing reaction to CO2 reduction, and improve the Faradaic efficiency (selectivity) for CO2 reduction (Figure 48). We also investigated the effects of temperature in the range of 20 °C to 90 °C. Our results showed that increasing the reactor temperature can significantly increase the rate of CO2 reduction. However, because of the decreased solubility of CO2 at higher temperatures, the performance quickly becomes mass-transfer limited and Faradaic efficiency decreases sharply (Figure 49). This work demonstrates the utility of the microfluidic reactor in studying a variety of parameters crucial to the efficient electrochemical reduction of CO2.
This research is supported by the Grainger Center for Electric Machinery and Electromechanics.